Dense smoke emitting from cars, dusty air hitting your face, or blackened building; these are just the signs showing you live in an area where most people are using cars without a catalytic convertor. Catalytic convertor is a device which is used to turn harmful chemical into harmless gases.
Catalytic Convertor:
Catalytic convertor basically breaks down the harmful gases which are harmless when they are independent or in ‘broken-molecule’ state. A catalyst is an element which creates a change in the reaction without actually breaking down itself. It does not react with the elements but enhances the reaction. In a catalytic convertor, palladium or rhodium is used as a catalyst.
How Catalytic Convertor Works:
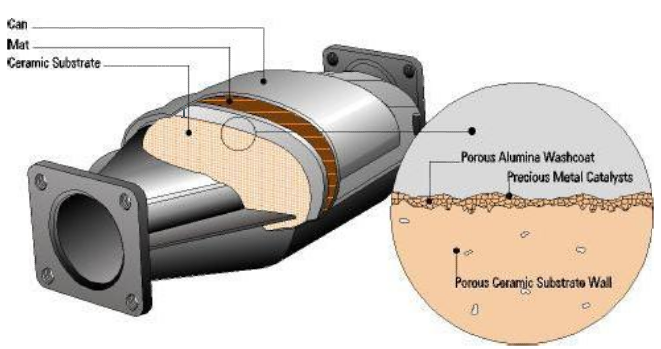
Catalytic convertor is situated under the vehicle which is directly attached to the engine. It has two tubes; one that takes in the contagious gases from the engine (input), other is attached to the tail pipe that emits out gas to the environment. The metal box is made of palladium, which acts as a catalyst. When the gases pass through the box, they break down into smaller molecules which are harmless.
Types of Catalytic Convertor:
Two way
A two-way catalytic converter generally has 2 tasks:
- oxidation of carbon monoxide to carbon dioxide: 2CO + O² → 2CO²
- Oxidation of hydrocarbons to carbon dioxide and water: CxH2x+2 + [(3x+1)/2] O2 → xCO2 + (x+1) H2O (a combustion reaction)
This convertor has a oxidation and combustion reaction occurring simultaneously.They are used in diesel engines to reduce hydrocarbon and carbon monoxide emissions. Their drawback was that they cannot break down oxides of nitrogen into nitrogen.
Three way
The drawback of two way catalytic convertor gave rise to 3 way catalytic converter. These are necessary in gasoline powered vehicles. In this convertor, oxidation and reduction occurs side by side.A three-way catalytic converter carries out three tasks:
- Reduction of nitrogen oxides to nitrogen and oxygen: 2NOx → xO2 + N2
- Oxidation of carbon monoxide to carbon dioxide: 2CO + O2 → 2CO2
- Oxidation of unburned hydrocarbons (HC) to carbon dioxide and water: CxH2x+2 + [(3x+1)/2] O2 → xCO2 + (x+1) H2O.
The car engine release contagious gases like oxides of nitrogen (NOx), carbon monoxide (CO) and Hydrocarbons (HC). These gases are extremely hazardous to the environment and fatal for human. A three way catalytic converter converts these harmful gases to harmless gases like nitrogen (N2), carbon-di-oxide (CO2) and water (H2O)
A three-way catalytic converter makes use of two catalysts to convert harmful gases to harmless gases. It consists of reduction Catalyst and an Oxidation Catalyst. Here the reduction catalyst is made of platinum and rhodium while the oxidation catalyst is made of platinum and palladium.
Diesel Engines
These convertors are 90 percent efficient. They are highly effective in eliminating diesel and in reduction of contagious particulate in the form of soot. However, they do not activate for oxides of nitrogen.
Lean Burn Spark Ignition Engines
A lean burn sparks ignition engines requires an oxidation catalyst. It works in just like diesel engine.
Author’s Biography:
David Russell works at catalytic exhaust in Canada. He has written number of articles on catalytic convertors also mentioning the catalytic convertors for sale.